Fda Generic Name Guidance . the draft guidance also describes the framework fda uses in evaluating proposed proprietary names for. approved brand name and generic drugs; this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in promotional labeling. this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in.
from aaos.org
this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in. approved brand name and generic drugs; this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in promotional labeling. the draft guidance also describes the framework fda uses in evaluating proposed proprietary names for.
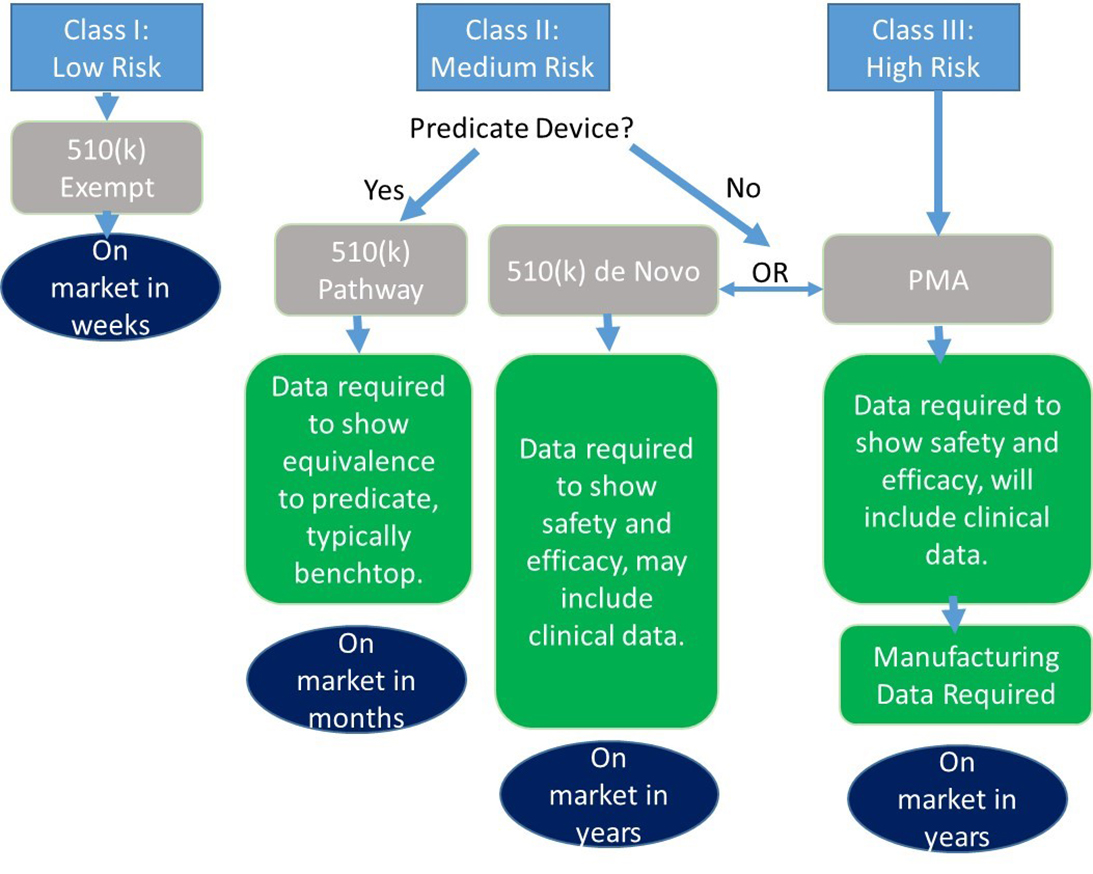
An Overview of the FDA Approval Process for Devices
Fda Generic Name Guidance this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in promotional labeling. the draft guidance also describes the framework fda uses in evaluating proposed proprietary names for. approved brand name and generic drugs; this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in promotional labeling. this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in.
From lubrizolcdmo.com
Key Takeaways From the FDA Complex Generic Drug Product Development Fda Generic Name Guidance this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in. the draft guidance also describes the framework fda uses in evaluating proposed proprietary names for. this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in promotional labeling. approved brand name and generic drugs; Fda Generic Name Guidance.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Fda Generic Name Guidance this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in promotional labeling. this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in. the draft guidance also describes the framework fda uses in evaluating proposed proprietary names for. approved brand name and generic drugs; Fda Generic Name Guidance.
From www.biopharmaservices.com
Bioequivalence & Bioavailability Studies BioPharma Services Fda Generic Name Guidance the draft guidance also describes the framework fda uses in evaluating proposed proprietary names for. this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in promotional labeling. approved brand name and generic drugs; this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in. Fda Generic Name Guidance.
From healthproadvice.com
What's the Difference Between Generic and BrandName Medicine Fda Generic Name Guidance approved brand name and generic drugs; this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in promotional labeling. this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in. the draft guidance also describes the framework fda uses in evaluating proposed proprietary names for. Fda Generic Name Guidance.
From www.youtube.com
FDA Pharmaceutical Validation Guidance and ICH What you must know Fda Generic Name Guidance this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in promotional labeling. the draft guidance also describes the framework fda uses in evaluating proposed proprietary names for. this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in. approved brand name and generic drugs; Fda Generic Name Guidance.
From wearephlo.com
Generic vs. brandname medication differences Phlo Blog Fda Generic Name Guidance this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in promotional labeling. the draft guidance also describes the framework fda uses in evaluating proposed proprietary names for. approved brand name and generic drugs; this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in. Fda Generic Name Guidance.
From feedmilling.ces.ncsu.edu
Now Available New FDA Guidance for Small Business Size NC State Fda Generic Name Guidance this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in. approved brand name and generic drugs; the draft guidance also describes the framework fda uses in evaluating proposed proprietary names for. this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in promotional labeling. Fda Generic Name Guidance.
From www.pharmaceutical-technology.com
FDA releases generics guidances to encourage competition Fda Generic Name Guidance this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in promotional labeling. the draft guidance also describes the framework fda uses in evaluating proposed proprietary names for. approved brand name and generic drugs; this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in. Fda Generic Name Guidance.
From www.keymkr.com
Was bedeutet die neue FDA Guidance Content of Human Factors Fda Generic Name Guidance this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in promotional labeling. the draft guidance also describes the framework fda uses in evaluating proposed proprietary names for. approved brand name and generic drugs; this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in. Fda Generic Name Guidance.
From www.mavenrs.com
Generics The FDA's Approval Track Record and New Guidance Drafts Maven Fda Generic Name Guidance this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in. this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in promotional labeling. approved brand name and generic drugs; the draft guidance also describes the framework fda uses in evaluating proposed proprietary names for. Fda Generic Name Guidance.
From www.researchgate.net
(PDF) An Overview on Bioequivalence Regulatory Consideration for Fda Generic Name Guidance this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in. the draft guidance also describes the framework fda uses in evaluating proposed proprietary names for. approved brand name and generic drugs; this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in promotional labeling. Fda Generic Name Guidance.
From infogram.com
Adderall Pill Identification Infogram Fda Generic Name Guidance this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in promotional labeling. approved brand name and generic drugs; the draft guidance also describes the framework fda uses in evaluating proposed proprietary names for. this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in. Fda Generic Name Guidance.
From insilicominds.com
FDA Launches General Principles of ModelIntegrated Evidence (MIE Fda Generic Name Guidance the draft guidance also describes the framework fda uses in evaluating proposed proprietary names for. this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in. approved brand name and generic drugs; this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in promotional labeling. Fda Generic Name Guidance.
From www.biochempeg.com
Evolution of GLP‐1 Receptor Agonists for Diabetes Treatment Biopharma PEG Fda Generic Name Guidance the draft guidance also describes the framework fda uses in evaluating proposed proprietary names for. approved brand name and generic drugs; this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in promotional labeling. this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in. Fda Generic Name Guidance.
From www.quantib.com
A 101 guide to the FDA regulatory process for AI radiology software Fda Generic Name Guidance approved brand name and generic drugs; this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in. the draft guidance also describes the framework fda uses in evaluating proposed proprietary names for. this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in promotional labeling. Fda Generic Name Guidance.
From vivafda.com
FDA Drug Labeling and Ingredient Requirement Viva FDA U.S. FDA Fda Generic Name Guidance this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in. this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in promotional labeling. the draft guidance also describes the framework fda uses in evaluating proposed proprietary names for. approved brand name and generic drugs; Fda Generic Name Guidance.
From healthmatch.io
HealthMatch Are Generic Drugs Just As Good As Branded Drugs? Fda Generic Name Guidance this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in. the draft guidance also describes the framework fda uses in evaluating proposed proprietary names for. approved brand name and generic drugs; this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in promotional labeling. Fda Generic Name Guidance.
From www.ezmedlearning.com
Antibiotic Class Chart and Drug Name List Pharmacology Mnemonic — EZmed Fda Generic Name Guidance the draft guidance also describes the framework fda uses in evaluating proposed proprietary names for. this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in. this guidance clarifies the requirements for product name placement, size, prominence, and frequency2 in promotional labeling. approved brand name and generic drugs; Fda Generic Name Guidance.